非酒精性脂肪性肝病(Nonalcoholic fatty liver disease, NAFLD)是一种脂肪在肝脏中过度累积的疾病。这种脂肪堆积不是由大量饮酒引起的。非酒精性脂肪性肝炎(Non-alcoholic steatohepatitis, NASH)是NAFLD的一种,除肝脏脂肪堆积外,还有肝炎、肝细胞损伤、纤维化及肝脏瘢痕形成的病理变化,进而可能导致肝硬化或肝癌。NASH进展的机制仍不清楚,目前还缺乏有效的治疗方法。
NASH的临床症状相当复杂,根据疾病过程包括肥胖、胰岛素抵抗、脂肪性肝炎、肝细胞气球样变和纤维化。临床前实验中经常使用的NASH模型有遗传动物模型、饮食诱导的动物模型及遗传与饮食诱导相结合构建的动物模型。然而,很难在短时间内模拟人类疾病的所有发病特征。
为了解NASH发生、发展的致病机制并开发创新疗法,我们开发了几种NASH不同发病阶段的小鼠模型。
1、西方饮食(WD):饮食成分中高脂、高果糖和高胆固醇,该饮食诱导的NASH模型发展为肥胖、糖耐量受损和肝脏脂肪变性。
2、高脂肪蛋氨酸胆碱缺乏饮食(HFMCD):饮食成分中含有60 kcal%脂肪,蛋氨酸、胆碱缺乏,该饮食诱导的NASH模型显示出肝损伤、肝脂肪变性和纤维化增加,伴随NAS评分增加。
3、四氯化碳(CCl4)注射:引起明显的肝脏炎症和纤维化。虽然很难获得完美的模型,但根据目标特点选择目前可用的合适模型是一个很好的选择。
HFMCD饮食是NASH的经典饮食模型。尽管饲料中含60 kcal%脂肪,但缺乏蛋氨酸和胆碱。蛋氨酸和胆碱都能促进脂肪以磷脂的形式由肝脏通过血液输送出去,提高脂肪酸在肝中的利用,防止脂肪在肝脏的异常堆积。
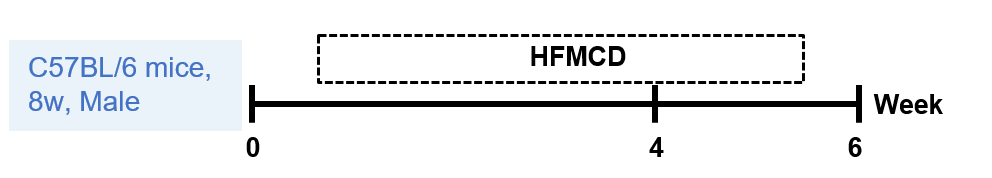
8周龄C57BL/6雄性小鼠分别给予标准饮食(STD)或高脂蛋氨酸胆碱缺乏饮食4或6周。
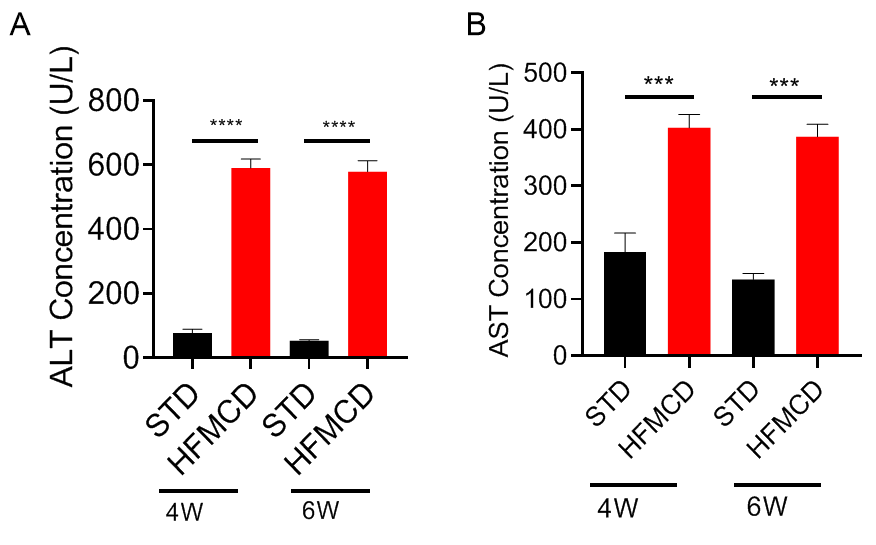
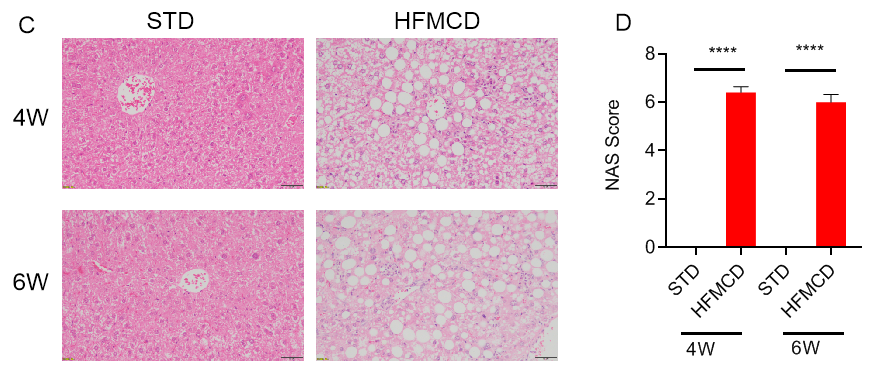
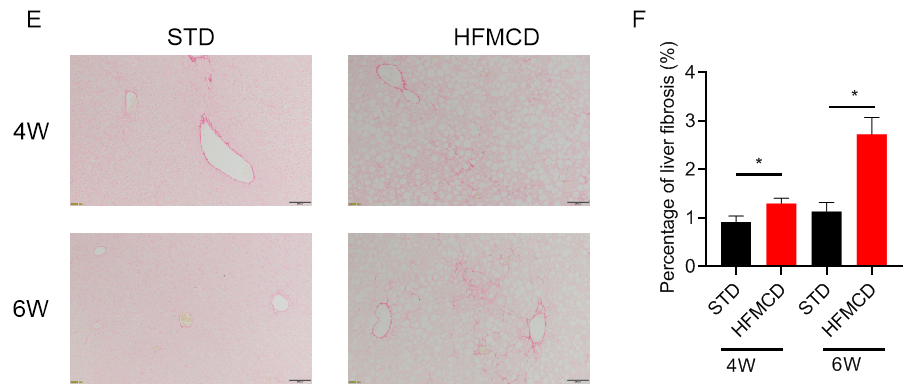
高脂肪蛋氨酸胆碱缺乏饮食(HFMCD)诱导的NASH模型。将野生型C57BL/6J小鼠随机分为4组,分别给予标准饲料(STD)和高脂肪蛋氨酸胆碱缺乏(HFMCD)饲料并喂养4周及6周后检测各项指标。(A-B)血清ALT、AST浓度;(C-D)肝组织切片HE染色及NAS评分;(E-F)肝组织切片天狼星红染色及肝纤维化程度统计。结果显示:HFMCD诱导组与STD组相比,肝脏重量明显增加;血清ALT、AST浓度显著升高;肝组织切片HE染色显示广泛的肝细胞脂肪变性、气球样变和小叶内炎症;肝组织天狼星红染色显示明显的肝纤维化。以上结果说明HFMCD诱导能成功建立NASH小鼠模型。数据为平均值±SEM,n = 5。
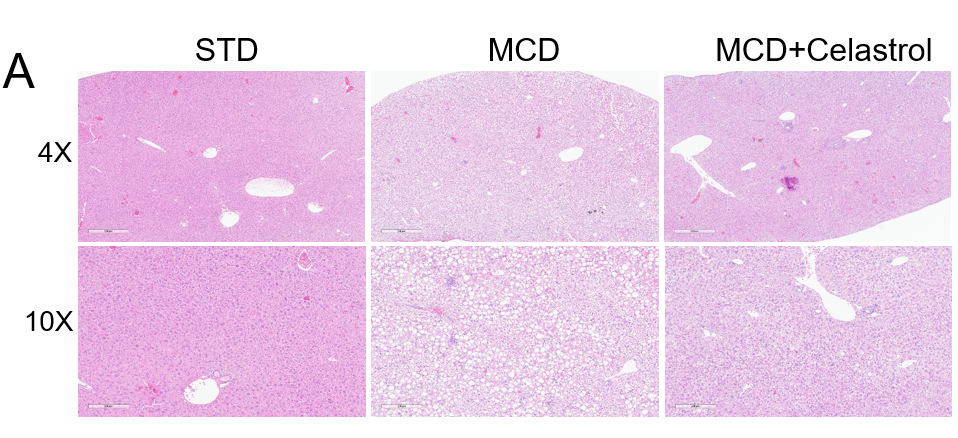
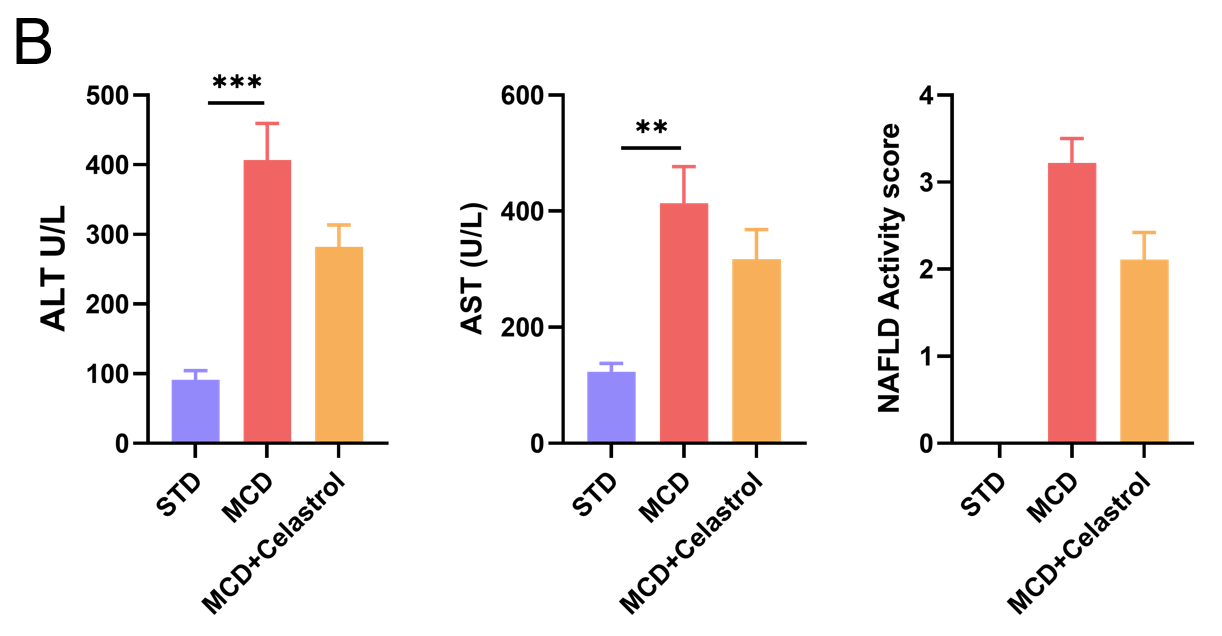
对HFMCD诱导的NASH小鼠模型的H&E染色、肝功能指数和NAFLD活性评分进行分析。(A) H&E染色的代表性图片显示celastrol治疗后肝脏脂肪变性和炎症减轻。(B) MCD诱导后ALT/AST变化。NAFLD活动评分(NAS)的统计数据。数值以平均值±SEM表示。**p<0.01,***p<0.001。
参考文献
1. Hernandez-Perez, E., Leon Garcia, P.E., Lopez-Diazguerrero, N.E., Rivera-Cabrera, F. & Del Angel Benitez, E. Liver steatosis and nonalcoholic steatohepatitis: from pathogenesis to therapy. Medwave 16, e6535 (2016).
2. Tsuchida, T., et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol 69, 385-395 (2018).
3. Farrell, G., et al. Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis. Hepatology 69, 2241-2257 (2019).
STAM-NASH模型构建
实验动物:C57BL/6小鼠,0-24h,雄性
造模试剂: High Fat Diet、 Streptozocin
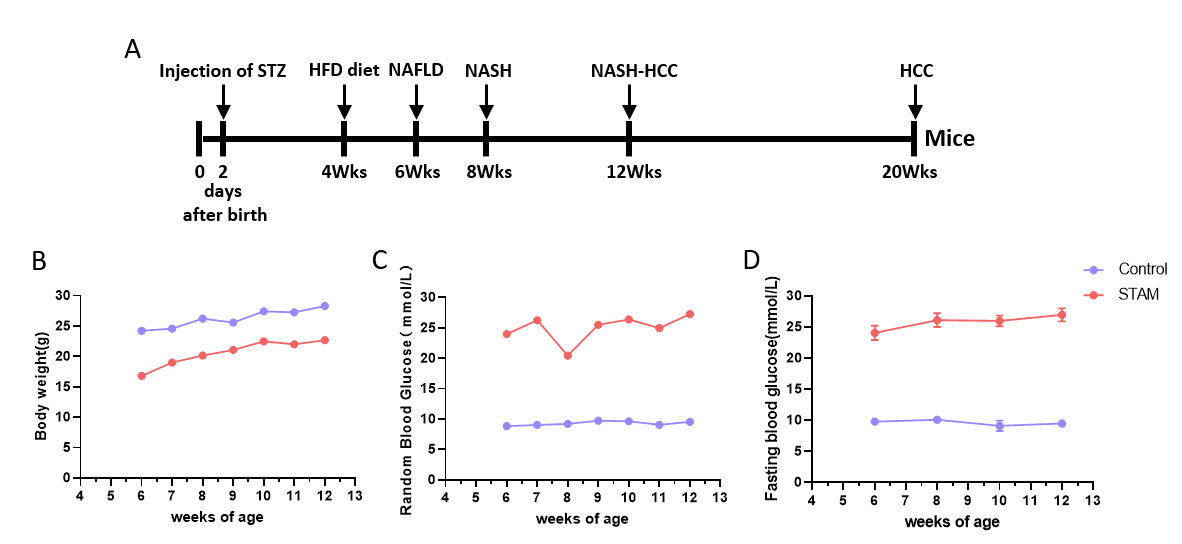
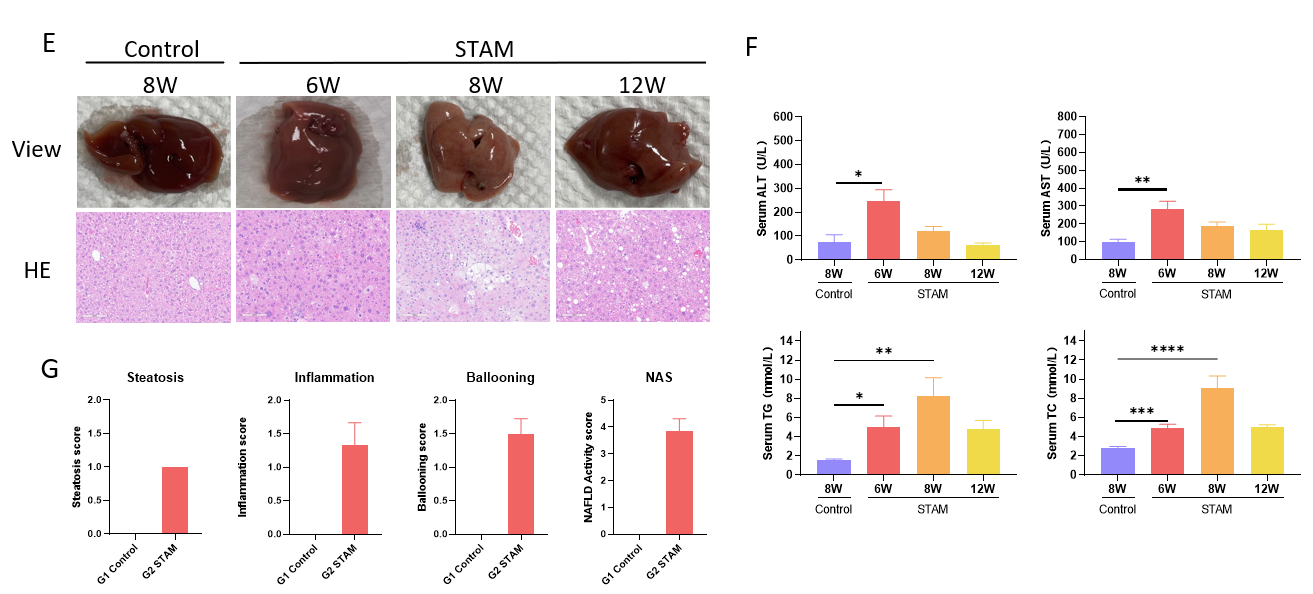
STAM (NASH-HCC) 模型小鼠构建与特征分析。(A) 新生雄性小鼠于第2天注射STZ, 4周龄开始喂食HFD饲料。(B-D) 诱导后体重和血糖变化。(E) 诱导后第6、8、12周HE染色代表性图片。(F) 血液生化结果显示STAM模型特征。(G) NAFLD活动评分。
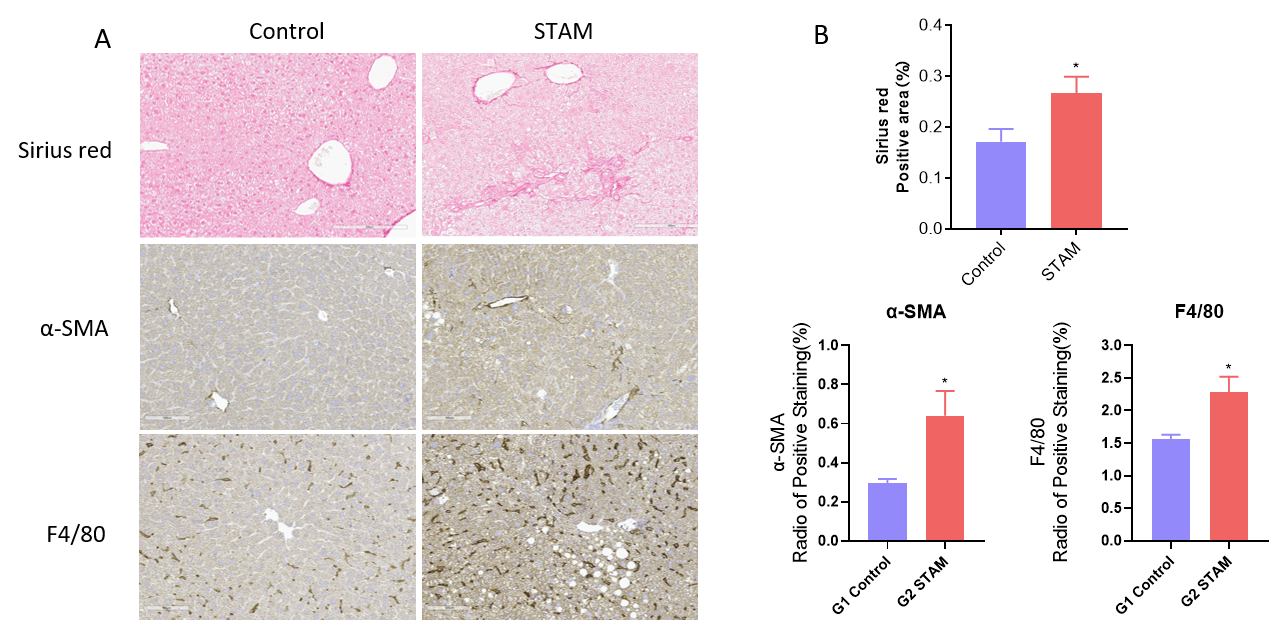
NASH-STAM模型的纤维化及炎症分析。(A)诱导9周后天狼星红和IHC染色的代表性图片。(B) 天狼星红和IHC染色定量数据。
一、C57BL/6老龄鼠的NASH模型
模型诱导
模型验证
GAN饮食诱导的老龄鼠NASH模型表现出明显的代谢紊乱
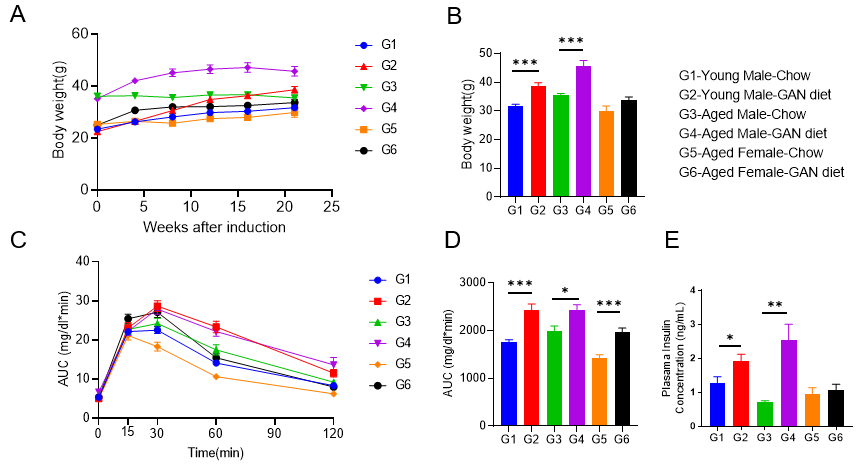
GAN饮食诱导的老龄鼠NASH模型表现出代谢紊乱。(A-B)GAN饮食诱导组的体重增加。(C)GAN饮食诱导组的葡萄糖耐受能力受损。(D)C图曲线下面积。(E)GAN饮食诱导组的血浆胰岛素含量增加。N=6-10 mice per group. Data are expressed as mean ± SEM. **: p<0.01.
GAN饮食诱导12周后,老龄鼠表现出比年轻小鼠更严重的NASH表型
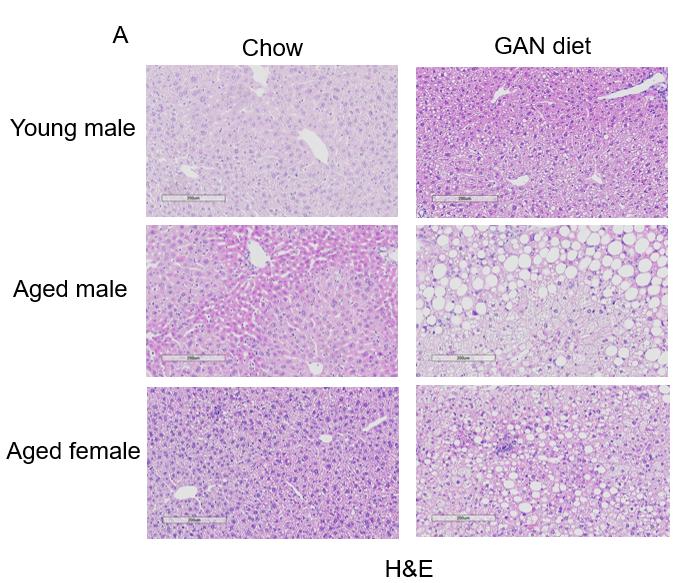

GAN饮食诱导的老龄鼠NASH模型。(A)诱导12周后H&E染色的代表性图片。(B)NAS(NAFLD acticity score)评分。
Data are expressed as mean ± SEM.N=6-10 mice per group. **: p<0.01.
GAN饮食诱导21周后,老龄鼠表现出比年轻小鼠更严重的纤维化
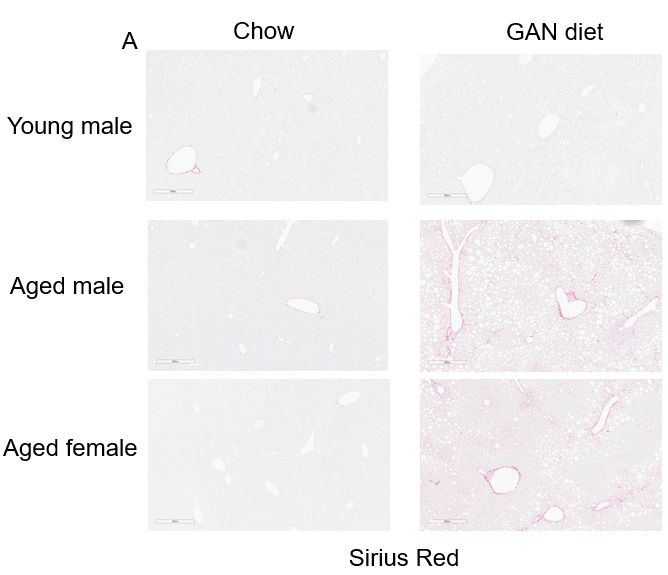
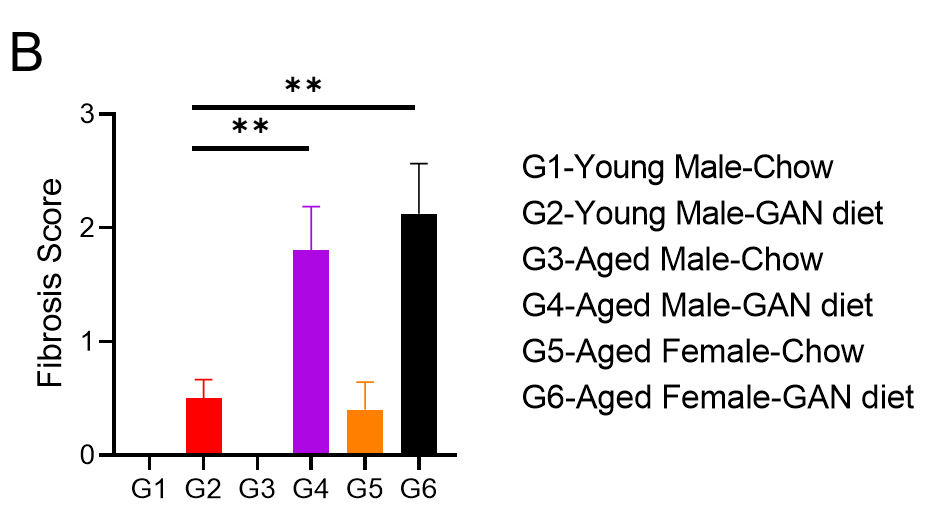
GAN饮食诱导的老龄鼠纤维化。(A)诱导21周后天狼星红染色的代表性图片。(B)天狼星红染色的纤维化评分。
Data are expressed as mean ± SEM. N=6-10 mice per group. **: p<0.01.
GAN饮食诱导21周后,老龄雄鼠肝脏中的免疫细胞浸润增加
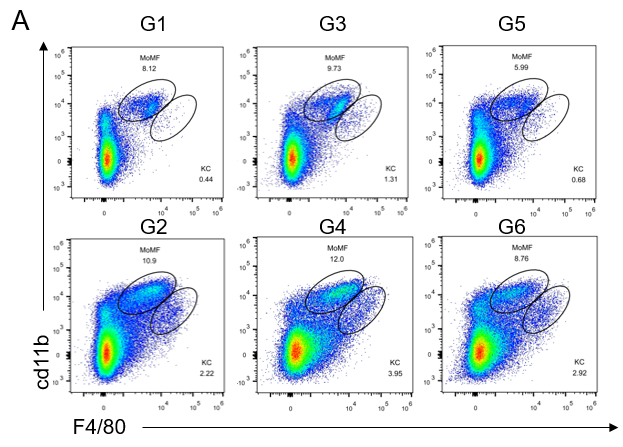
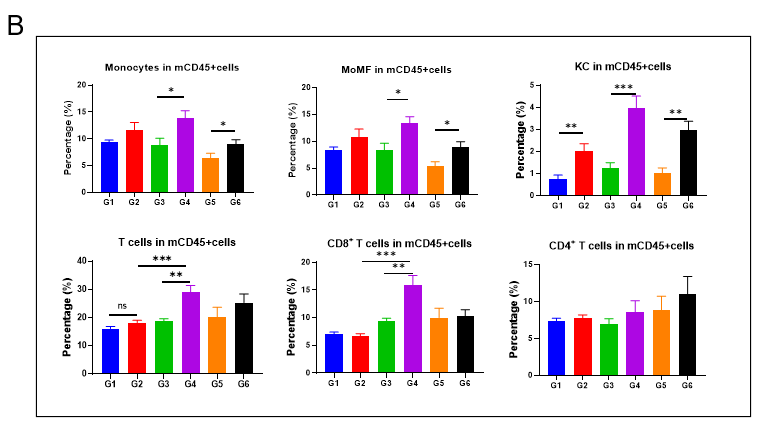
GAN饮食诱导21周后,老龄雄鼠肝脏中的免疫细胞浸润增加。(A)流式细胞仪评估肝脏中单核细胞(CD11bintF4 / 80low)和kupffer细胞(CD11b+F4 / 80hi)浸润比例。(B)肝脏中不同类型免疫细胞分析。
Data are expressed as mean ± SEM. N=6-10 mice per group. *P <0.05, **P <0.01, ***P <0.001.
模型诱导
模型验证
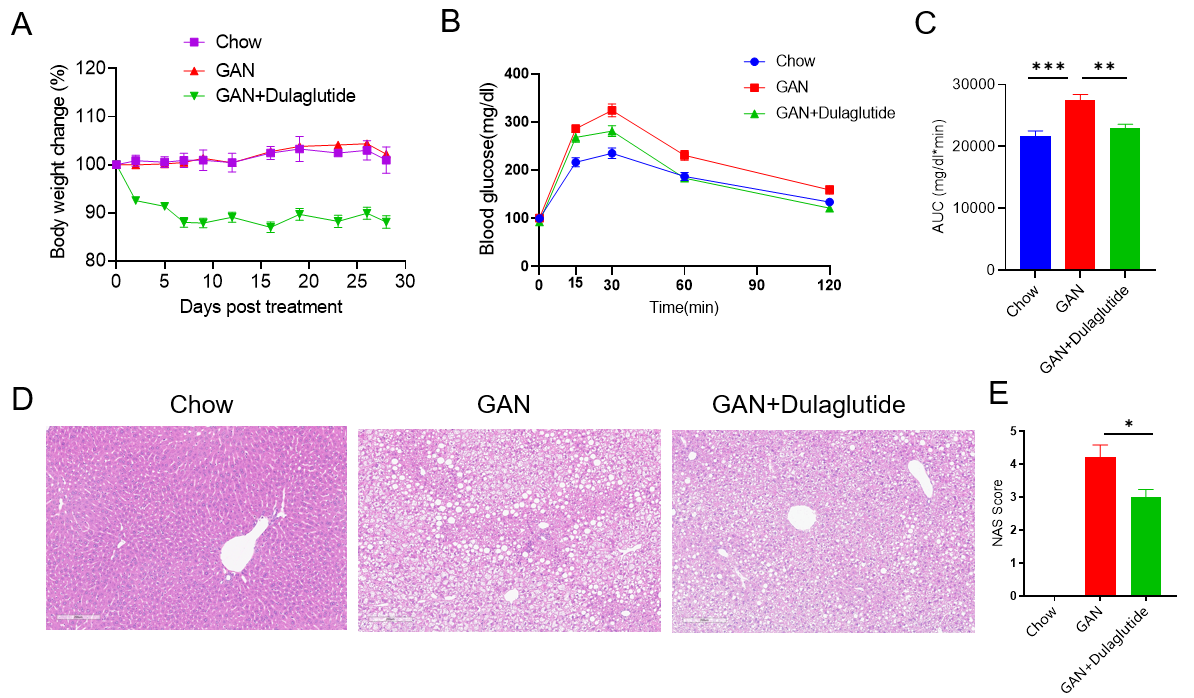
GAN饮食诱导的C57BL/6小鼠NASH模型。(A)治疗下的体重变化。(B)治疗后的葡萄糖耐受能力。(C)B图曲线下的面积。(D)诱导20周后H&E染色的代表性图片。(E)NAS(NAFLD acticity score)评分。
Data are expressed as mean ± SEM. N=9 mice per group. *p<0.05, **p<0.01,***p<0.001.
模型构建
模型验证
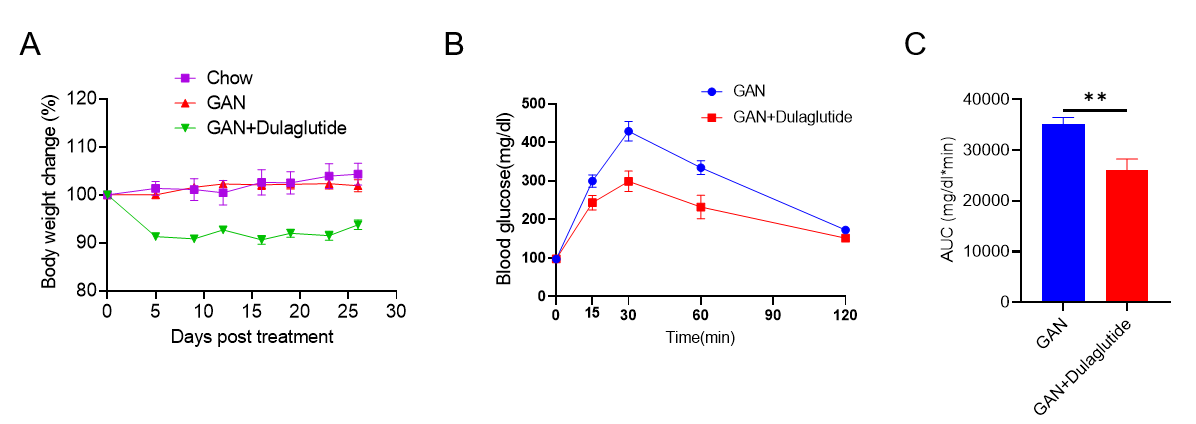
GAN饮食诱导的B-ob/0b小鼠NASH模型。(A)治疗下的体重变化。(B)治疗后的葡萄糖耐受能力。(C)B图曲线下的面积。
Data are expressed as mean ± SEM. N=9 mice per group. *p<0.05, **p<0.01,***p<0.001.
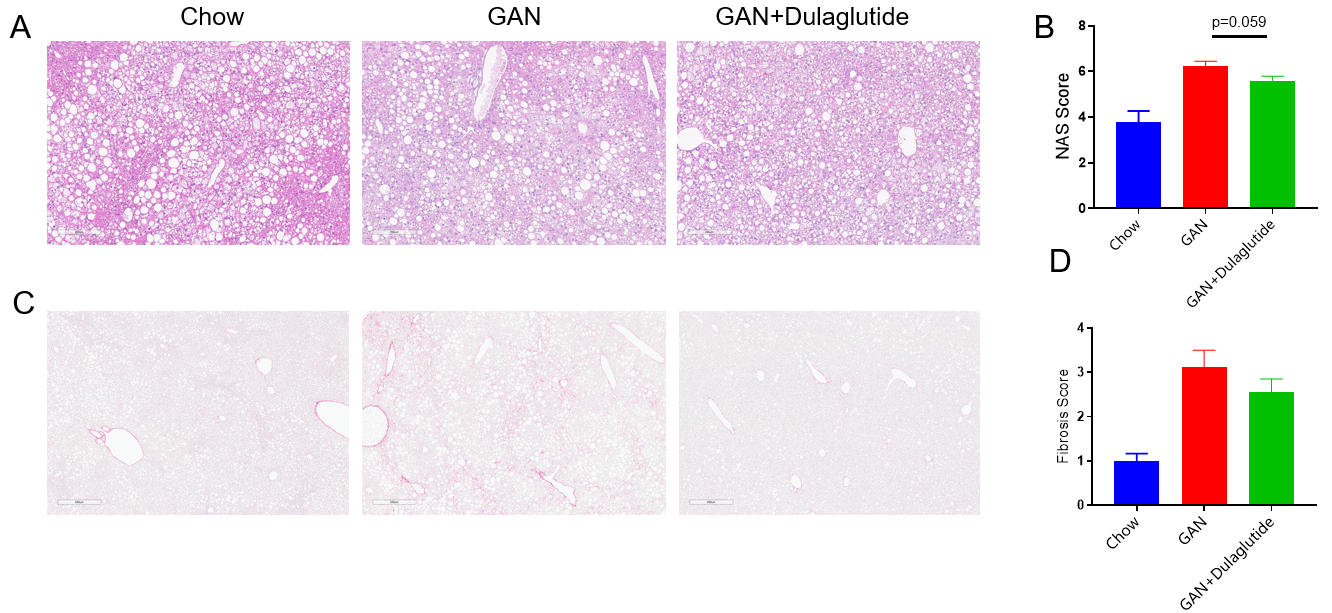
GAN饮食诱导B-ob/ob小鼠的NASH及纤维化。(A)诱导16周后H&E染色的代表性图片。(B)NAS(NAFLD acticity score)评分。(C)诱导16周后天狼星红染色的代表性图片。(D)天狼星红染色的纤维化评分。
Data are expressed as mean ± SEM. N=9 mice per group.
参考文献
1. Hansen, H.H., et al., Human translatability of the GAN diet-induced obese mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020 Jul 6;20(1):210.
2 .Hansen, H.H., et al., Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov Today, 2017. 22(11): p. 1707-1718.
3. Ibrahim, S.H., et al., Animal Models of Nonalcoholic Steatohepatitis: Eat, Delete, and Inflame. Dig Dis Sci, 2016. 61(5): p. 1325-36.
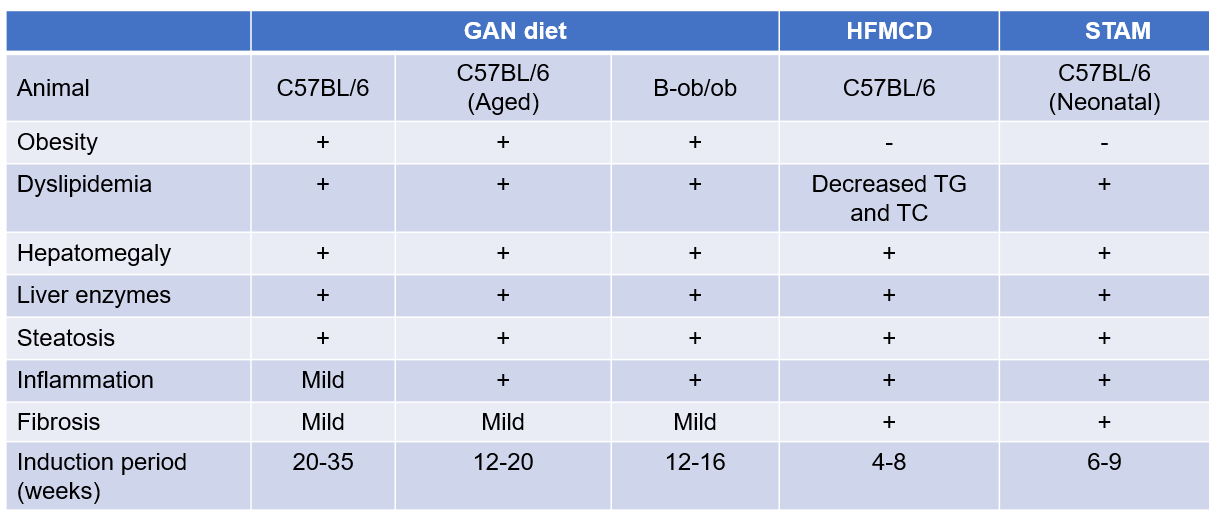
饮食和化学诱导的NASH小鼠模型比较
|
特征 |
WD |
HFMCD |
CCl4 |
|
肥胖 |
+ | - | - |
|
血脂异常 |
+ |
TG、TC降低 |
- |
|
肝肿大 |
+ | + | + |
|
肝转氨酶 |
+ | + | + |
|
脂肪变性 |
+ | + | - |
|
炎症 |
轻微 |
+ | + |
|
纤维化 |
轻微 |
+ | + |
|
诱导时间 (周) |
12-24 | 4-8 | 0.5-4 |






 010-56967680
010-56967680 info@bbctg.com.cn
info@bbctg.com.cn